-
Posts
917 -
Joined
-
Last visited
-
 Valeriy V reacted to a post in a topic:
ZULU 1916 by Ras Ambrioso - 1/48 scale - stern paddlewheeler
Valeriy V reacted to a post in a topic:
ZULU 1916 by Ras Ambrioso - 1/48 scale - stern paddlewheeler
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Canute reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
mtaylor reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
It is better to impregnate paper with cyacrine glue, but several layers are needed for good strength. I wish you success in your experiments!
-
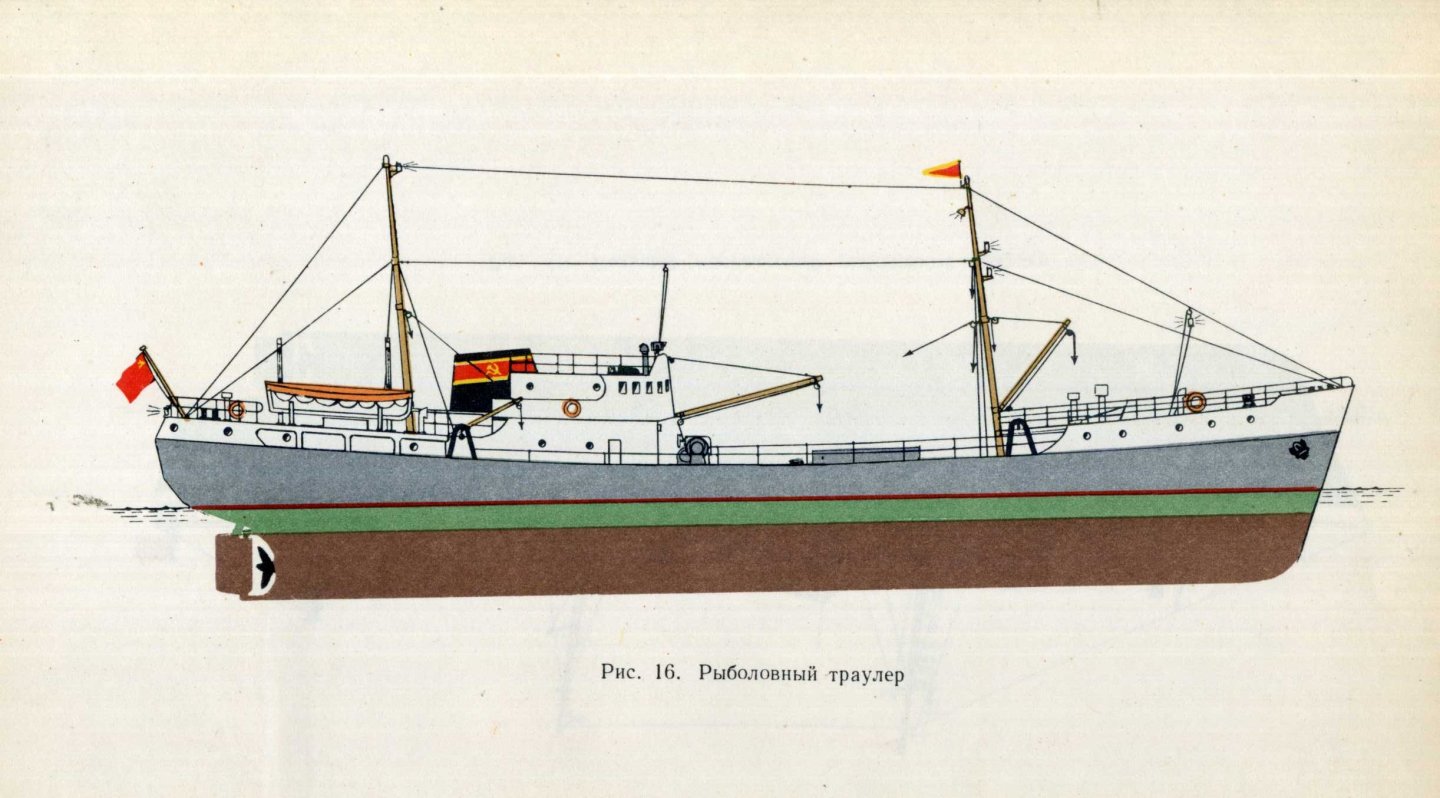
The rules for painting fishing vessels also include an option with a black freeboard. But to my taste the gray color looks more attractive.
-
 Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
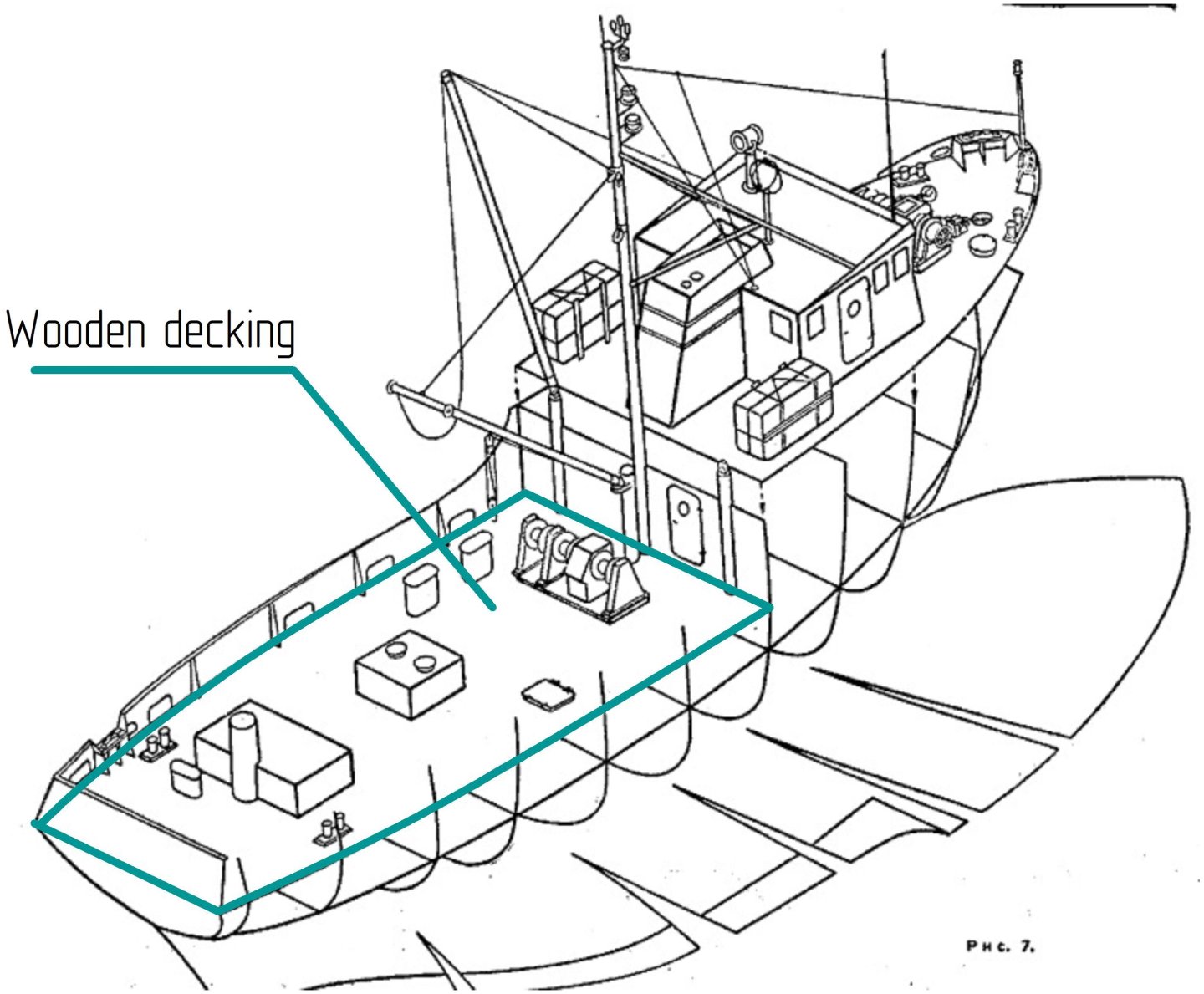
If I understand you correctly, on trawlers there was a wooden flooring only in the deck area where they directly worked with the fish catch.
-
If you cover the case with only one layer of epoxy resin without reinforcing it with any material, then after drying the resin will crack and fall off the case. If you cannot work with fiberglass, then it can be replaced with ordinary loose fabric like a medical bandage or gauze. But for epoxy resin, a reinforcement layer is required.
-
David! The ships of the USSR fishing fleet were painted according to their own rules. I am attaching a file with a painting scheme for trawlers. It is taken from a document entitled “Rules for painting vessels of the USSR fishing industry fleet” approved by the USSR Ministry of Fisheries on 08.28.69.
-
 Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
David, if you don't coat your hull with a layer of fiberglass and epoxy resin, it's likely to warp and crack. Putty will not be able to protect him from this process.
-
Hello David! This shrimp trawler was built according to Project 1329 in a single copy. It was called "Krevetka" (in Russian this is shrimp). https://rybflot.com/rybolovnyj-flot-sssr/dobyvayushhie-suda/malotonnazhnye/malyj-krevetkolovnyj-trauler-morozilnyj-tipa-krevetka-proekt-1329/ https://rybflot.com/wp-content/uploads/MKTM-tipa-Krevetka.pdf Eight more ships of this type were built a little later according to Project 1336 (Omar type). https://rybflot.com/rybolovnyj-flot-sssr/dobyvayushhie-suda/malotonnazhnye/malyj-krevetkolovnyj-trauler-morozilnyj-tipa-omar-proekt-1336/ Initially, these ships were built for a customer from Kuwait, but it turned out that he was insolvent and they were subsequently completed for the USSR Ministry of Fisheries.
-
 Valeriy V reacted to a post in a topic:
Steam Yacht Cangarda 1901 by KeithAug - Scale 1:24 - 1901/2008
Valeriy V reacted to a post in a topic:
Steam Yacht Cangarda 1901 by KeithAug - Scale 1:24 - 1901/2008
-
 Valeriy V reacted to a post in a topic:
85' ARB by Melissa T. - Scale 1:32 - POB - First Scratch Build
Valeriy V reacted to a post in a topic:
85' ARB by Melissa T. - Scale 1:32 - POB - First Scratch Build
-
 Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
Valeriy V reacted to a post in a topic:
Krevetka by doivud - CARD - Soviet shrimp trawler - First Build
-
 Valeriy V reacted to a post in a topic:
SS Blagoev (ex Songa )1921 by Valery V - scale 1:100 - Soviet Union
Valeriy V reacted to a post in a topic:
SS Blagoev (ex Songa )1921 by Valery V - scale 1:100 - Soviet Union
-
 Valeriy V reacted to a post in a topic:
Lightship ELBE 1 by Mirabell61 - scale 1:87 - launched 1948
Valeriy V reacted to a post in a topic:
Lightship ELBE 1 by Mirabell61 - scale 1:87 - launched 1948
-
 Valeriy V reacted to a post in a topic:
Lightship ELBE 1 by Mirabell61 - scale 1:87 - launched 1948
Valeriy V reacted to a post in a topic:
Lightship ELBE 1 by Mirabell61 - scale 1:87 - launched 1948
-
 Valeriy V reacted to a post in a topic:
Steam Yacht Cangarda 1901 by KeithAug - Scale 1:24 - 1901/2008
Valeriy V reacted to a post in a topic:
Steam Yacht Cangarda 1901 by KeithAug - Scale 1:24 - 1901/2008
About us
Modelshipworld - Advancing Ship Modeling through Research
SSL Secured
Your security is important for us so this Website is SSL-Secured
NRG Mailing Address
Nautical Research Guild
237 South Lincoln Street
Westmont IL, 60559-1917
Model Ship World ® and the MSW logo are Registered Trademarks, and belong to the Nautical Research Guild (United States Patent and Trademark Office: No. 6,929,264 & No. 6,929,274, registered Dec. 20, 2022)
Helpful Links
About the NRG
If you enjoy building ship models that are historically accurate as well as beautiful, then The Nautical Research Guild (NRG) is just right for you.
The Guild is a non-profit educational organization whose mission is to “Advance Ship Modeling Through Research”. We provide support to our members in their efforts to raise the quality of their model ships.
The Nautical Research Guild has published our world-renowned quarterly magazine, The Nautical Research Journal, since 1955. The pages of the Journal are full of articles by accomplished ship modelers who show you how they create those exquisite details on their models, and by maritime historians who show you the correct details to build. The Journal is available in both print and digital editions. Go to the NRG web site (www.thenrg.org) to download a complimentary digital copy of the Journal. The NRG also publishes plan sets, books and compilations of back issues of the Journal and the former Ships in Scale and Model Ship Builder magazines.